Cell Line Development (CLD) is a crucial first step in the process of producing your recombinant proteins, biologics, or monoclonal antibodies (mAbs). It is therefore essential to choose a partner with extensive cell culture expertise and powerful gene editing technologies. Today, the cell line development field faces challenging directives, which includes production of complex biologics such as engineered bispecific antibodies (BsAbs) and other difficult to express proteins.
At Hera, we focus on engineering mammalian cells, such as CHO cells (Chinese hamster ovary) or HEK-293 cells, to generate high expressing/high titer clones that are optimized for scale-up for research-grade or GMP bioproduction.
Our gene editing platform is ideal for producing your protein whether it is for human therapeutics, animal health, diagnostic, or research applications.
Harnessing piggyBac for Antibody Production in CHO Cells
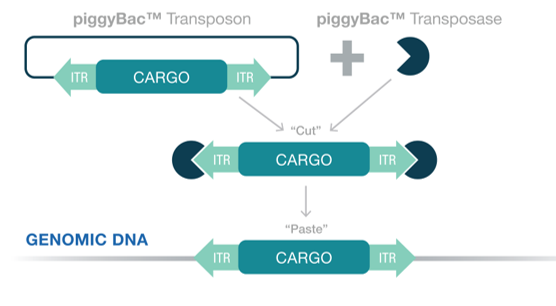
Creating stable cell lines with piggyBac
PiggyBac offers a robust and efficient method for the generation of stable CHO cell lines for high-yield antibody production. This system allows the integration of the antibody gene of interest into the CHO cell genome, ensuring its continuous expression.
1. High Efficiency: Generating stable pools with piggyBac is more efficient, reducing the time and cost involved in the production of therapeutic antibodies.
2. High Integration Capacity: PiggyBac can carry large DNA fragments, allowing for the co-expression of multiple genes which is especially beneficial for producing bispecific or multivalent antibodies.
3. Stability: The piggyBac system provides stable, long-term expression of the antibody, even over extended culture periods and which is crucial for the scale-up process and production of antibodies.
Achieve high titers and long term stability with piggyBac transposon DNA delivery system
The piggyBac transposon DNA delivery technology is the gold standard for expressing antibodies or proteins of interest for bioprocess used by pharmaceutical companies around the world to produce proteins at every stage of the drug development process. In addition to being extremely stable, piggyBac enables extremely high titers from early discovery to therapies in the clinic. Eli Lilly showed that, with piggyBac transposons, you can achieve pools of CHO cells with high titers and extreme stability over 55 doublings without having any decrease in productivity of the cells.
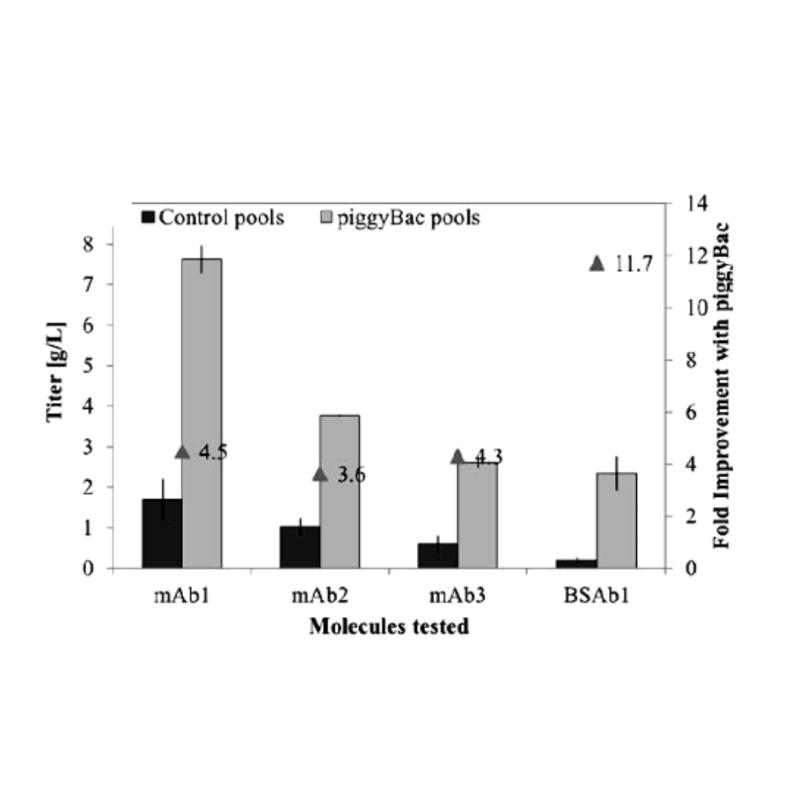
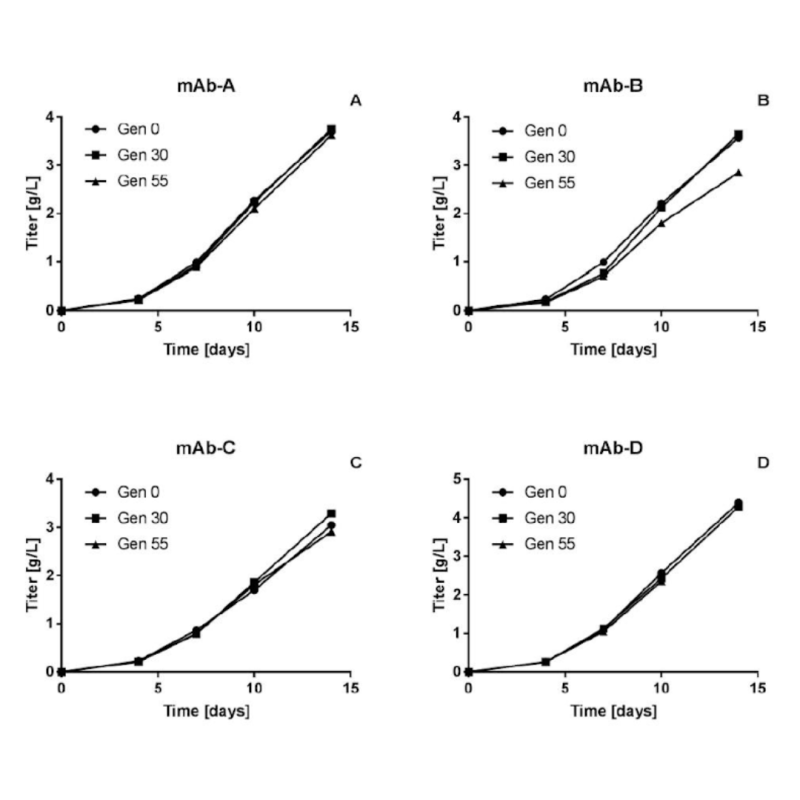
Rajendra, Y., Peery, R. B., & Barnard, G. C. (2016). Generation of stable Chinese hamster ovary pools yielding antibody titers of up to 7.6 g/L using the piggyBac transposon system. Biotechnology progress, 32(5), 1301–1307. https://doi.org/10.1002/btpr.2307
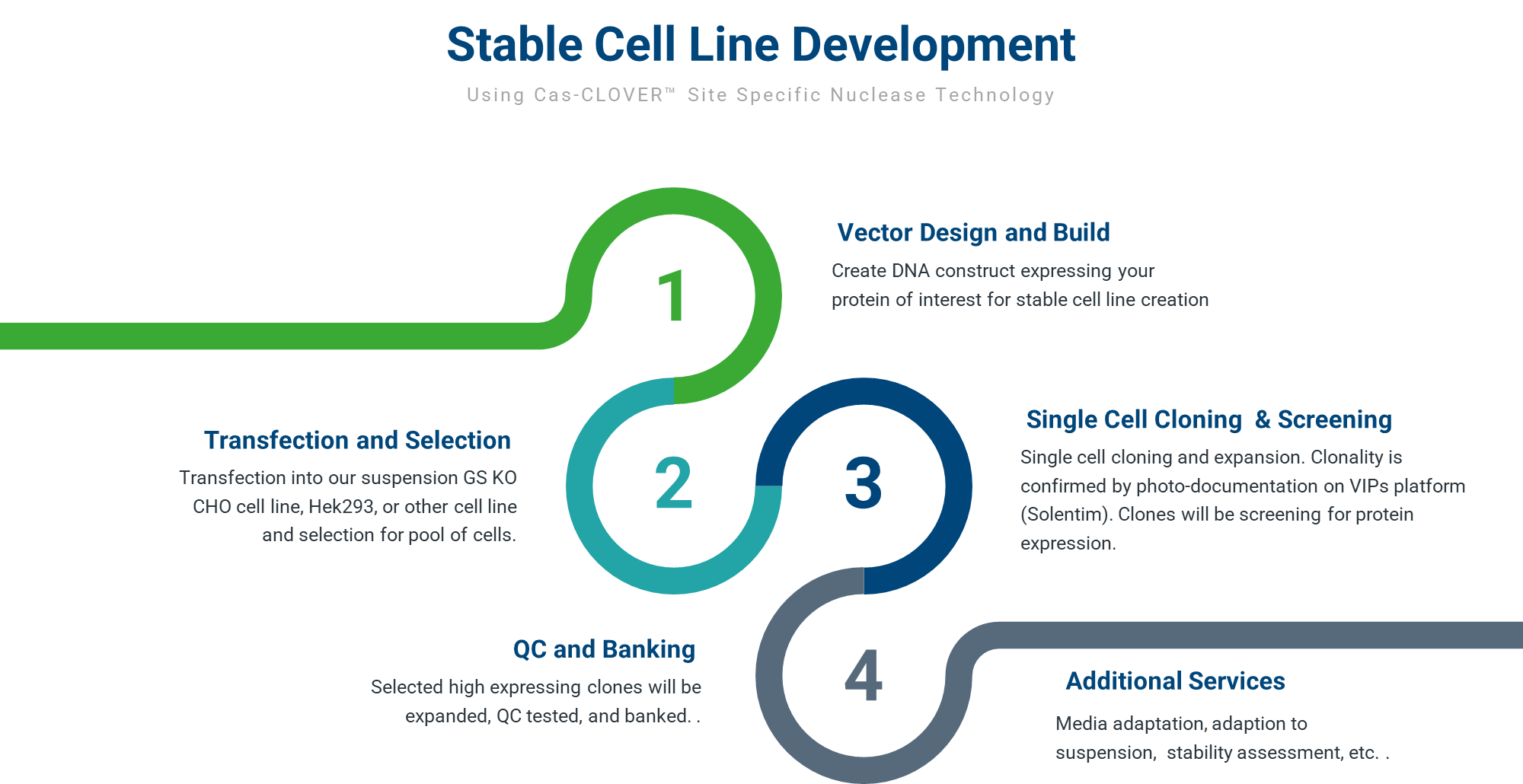
Cell Line Development Process
Our cell line development process starts with designing and building the gene editing reagents and donor plasmid containing the protein expression construct. This is followed by transfection, GS selection, and single cell cloning.
Clones are then screened for protein expression and growth characteristics. Our process allows us to obtain accurate titer measurements and rank clone productivity. As part of our standard service offering, we provide up to 10 clones that meet client requirements. Monoclonal lines are then expanded, QC tested, and are stored as research cell banks (RCBs).
From small biotech to large pharma companies, most find the cell line development process fraught with challenges, as well as, tedious and time-consuming work, leading to increased outsourcing at the CLD stage of protein production to specialized CROs like Hera.
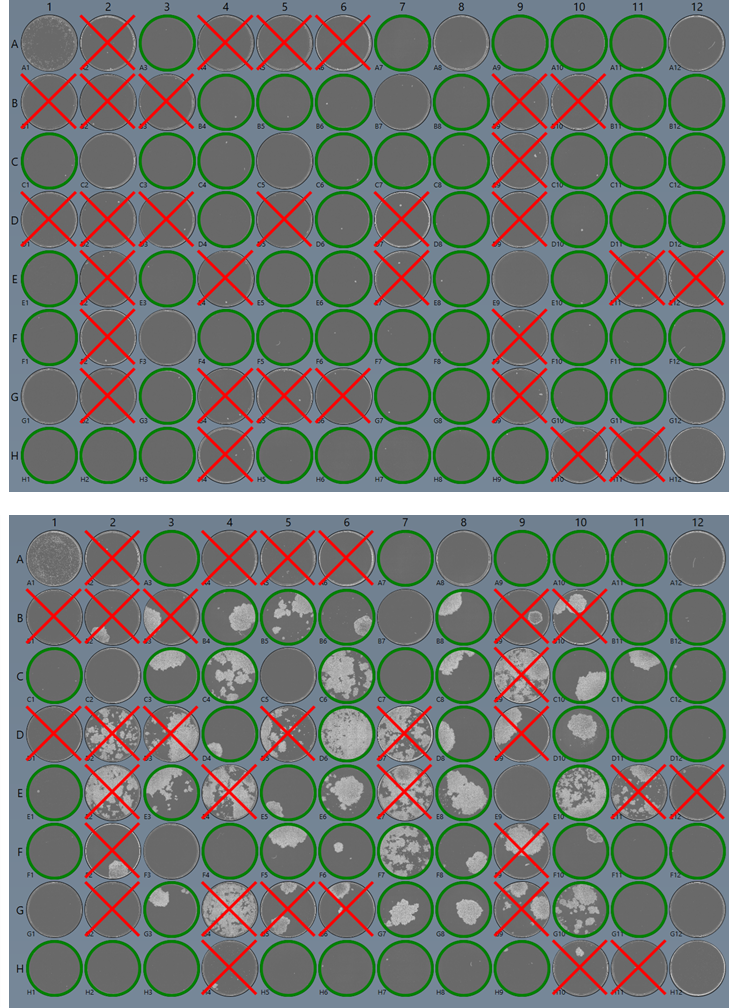
Single Cell Cloning
Demonstrating that your CHO cells are monoclonal is a regulatory requirement. Hera confirms clonality with imaging/visual photo-documentation with the Solentim VIPS™ (verified in-situ plate seeding) platform designed to provide industry standard image-based evidence of clonal derivation. VIPS dispenses cells in nanoliter droplets into microplate wells. The cell is imaged in the bottom of the dry well as evidence of clonal seeding. Automated imaging in multiple tight z-stack layers of the whole droplet combines with intelligence-based image analysis to confirm single cell seeding. The well is then filled with growth media. From the day of seeding and throughout the clonal growth phase, VIPS performs daily high clarity whole-well imaging, recording a timeline of well images and performing analysis to measure confluency.
Successful clonal outgrowth can be traced back to a single cell both at the seeding event and the first whole-well image to provide dual lines of monoclonality evidence.
Protein Expression and Stable Cell Lines
As indications for biologic drugs expand and the demand for biosimilars grow, improving drug development and manufacturing processes is increasingly important. We have demonstrated Cas-CLOVER to be a high efficiency targeted gene editing tool for use in HEK293 and CHO cells, base cell-lines validated for bioproduction.
Our cell line optimization methods are further streamlined using automated workflows for single-cell cloning with image-based documentation. Our Cas-CLOVER technology is essential for researchers looking for a genome editing technology with a clear freedom to operate optimized for antibody production.
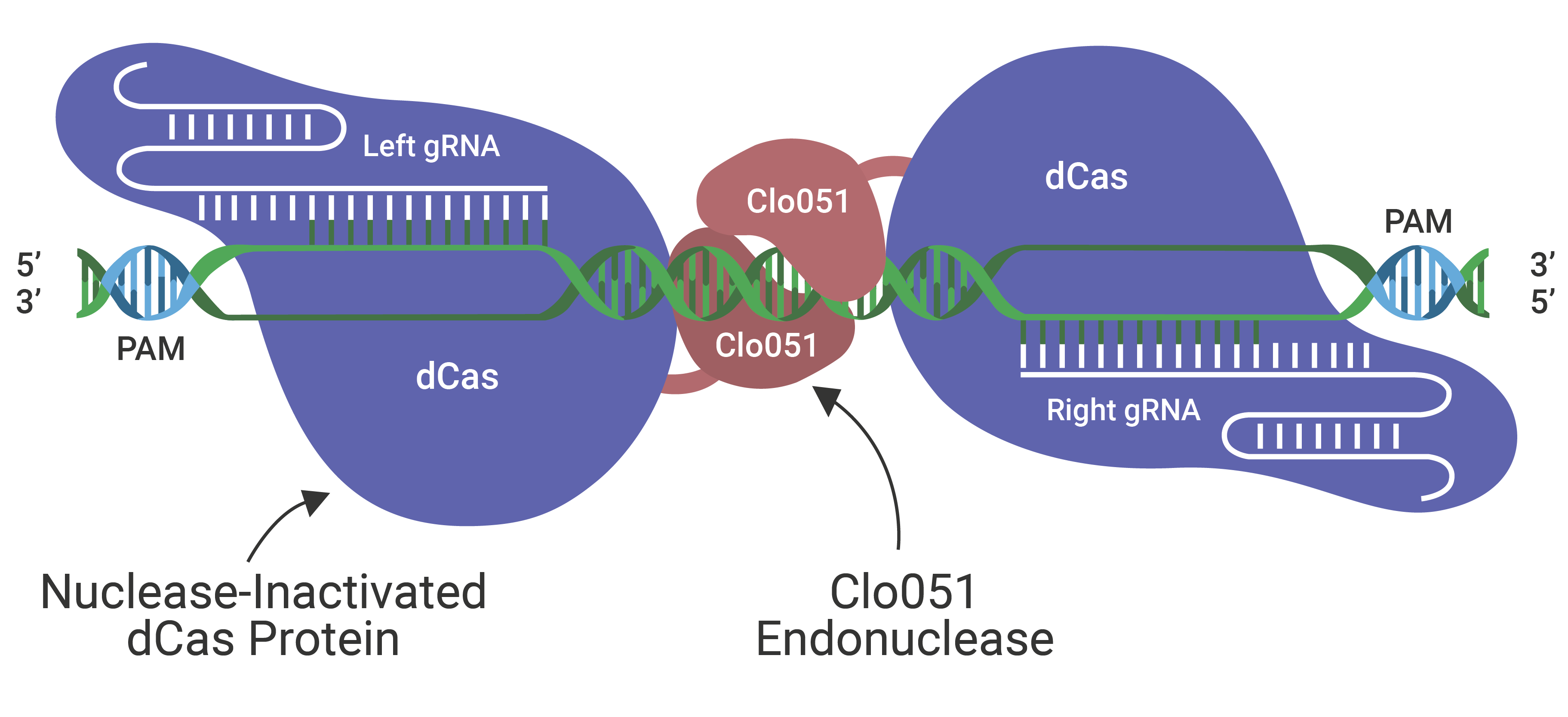
Cas-CLOVER TM
The Cas-CLOVER Site Specific Nuclease Technology is our highly specific dimeric nuclease technology for targeted gene modifications. With this technology, we engineered our GS (glutamine synthase) knockout suspension CHO cell line.
Cas-CLOVER has been optimized for high efficiency on-target nuclease activity with low to zero off target mutagenesis. The increased specificity is the result of using a pair of gRNAs and the obligate dimer CLO-51 for the enzymatic activity.
We have freedom to operate using our proprietary patented Cas-CLOVER technology, allowing Hera to provide custom cell line development services with flexible and clear commercial terms.
Cas-CLOVER for Host Cell Modifications and Protein Expression
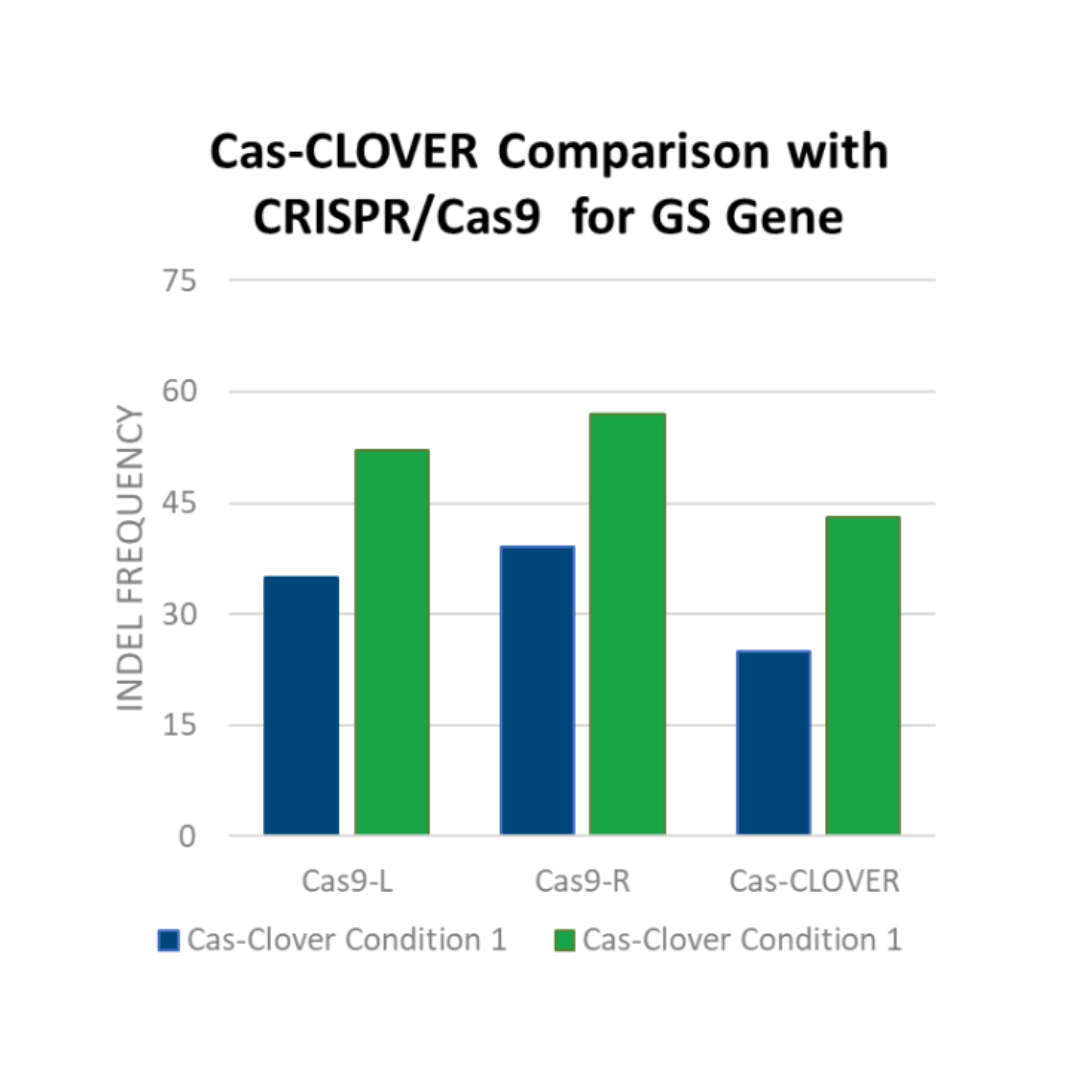
Hera has used Cas-CLOVER to create cell lines with highly sought modifications, such as fucosyltransferase 8 knockout (Fut8 KO) and glutamine synthetase knockout (GS KO) to engineer host cell lines to allow for identification of stable, high-expressing CHO cell clones. Efficient production of our base CHO cells lacking the GS gene is shown in the figure to the right.
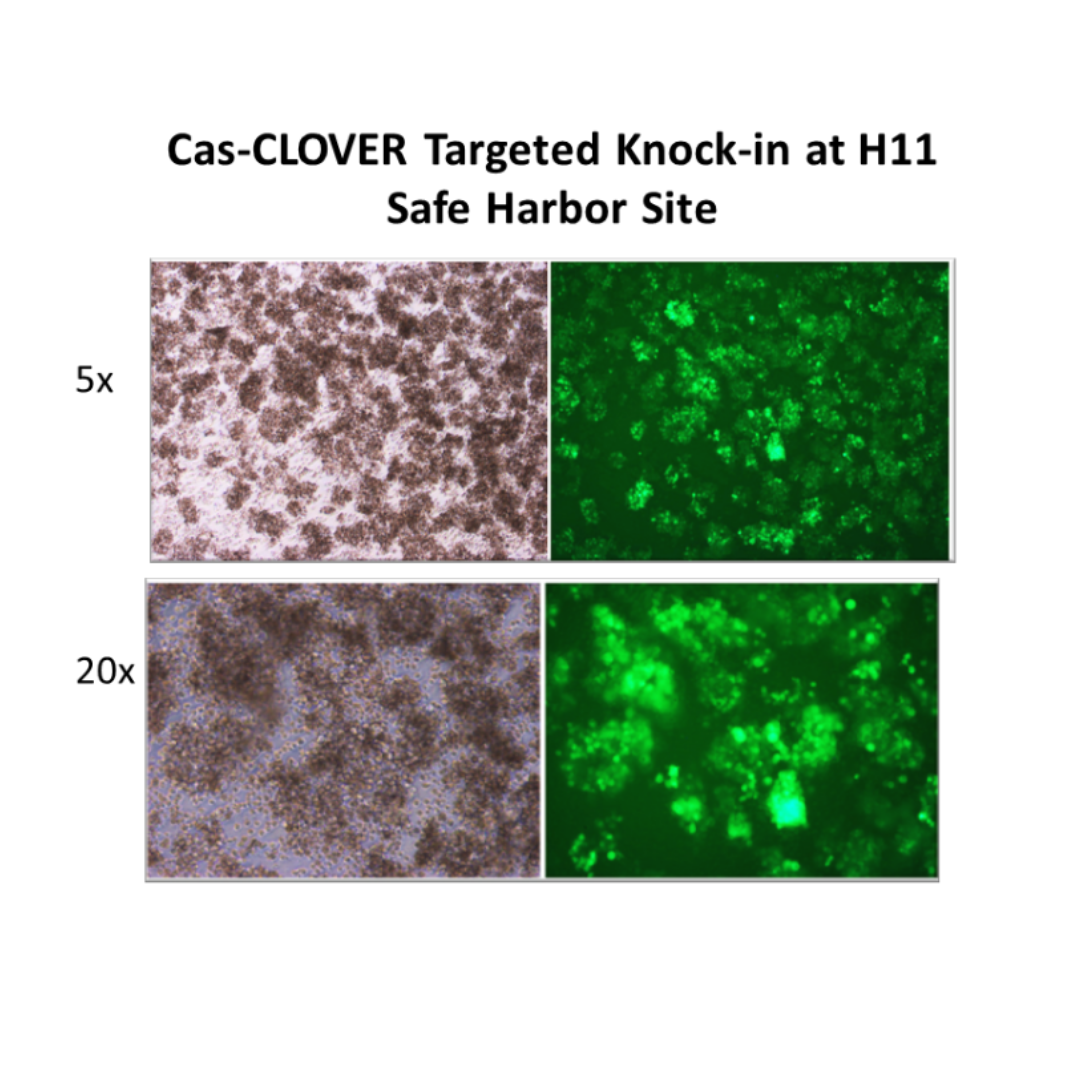
Knock-ins with Safe Harbor Loci
We also use proprietary technology for the targeted knock-in of human genes to safe harbor loci of cell lines. This enables stable and predictable overexpression of proteins of interest and avoids negatively affecting cellular characteristics.
For example, our scientists have developed optimized Cas-CLOVER reagents and protocols for targeting at the Hipp11 safe harbor locus in CHO suspension cultures that show extremely high target knock-in (80-90%) efficiency (see figure to the left).
Stable Expression vs. Transient Transfections
One approach to antibody production is to transiently express the protein of interest. However, the platform cell lines (CHO or HEK293) lose expression after 10-12 passages. For producing antibodies reproducibility and with the ability to scale, it is important to engineer stable cell lines in which the expression construct is integrated into the genomic DNA.
Our Gene Editing Technology
Hera’s cutting-edge piggyBac gene delivery technology and Cas-CLOVER site specific nuclease are an ideal combination for tackling your most challenging cell line engineering projects whether it is reporter cell lines, custom assays, or Cell Line Development. Learn more about these technologies by clicking the links below.