In Vivo Gene Delivery To The Liver
Combining the piggyBac™ transposon system with viral technology for in vivo stable transgene delivery to the rat liver
The in vivo delivery of transgenes into the liver of rats provide a rapid method for the creation of liver specific disease models, as well as gene therapy efficacy and toxicity testing models, without the time and cost for embryo modification and transgenic animal creation. By combining the highly efficient liver-targeting properties of rAAV with the piggyBac transposon, the transgene of interest stably integrates into the genome and is reliably expressed1.
Liver disease and gene therapy rodent model creation and screening services without the time and expense of making transgenic animals
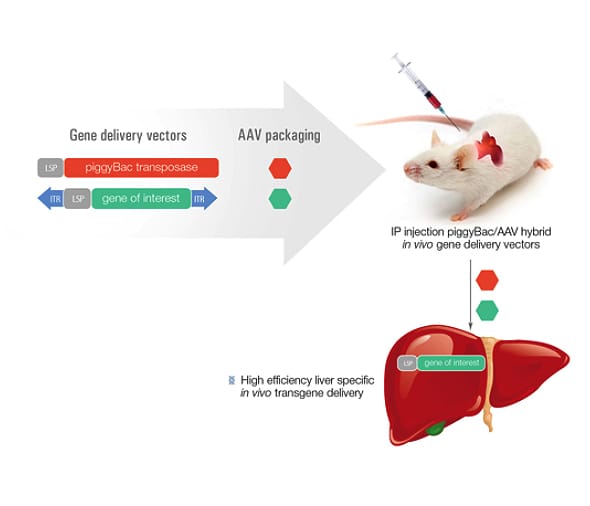
Figure 1: How the piggyBac/AAV delivery system works
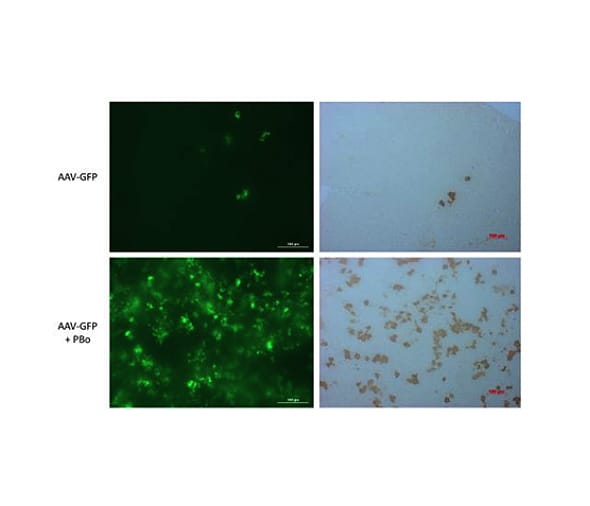
Figure 2: Example In Vivo Data
Neonatal rats were injected with rAAV at 1.5 days old. Rats received rAAV (5e10 vector genomes) expressing GFP with or without rAAV (5e10 vector genomes) expressing piggyBac transposase (without piggyBac, top; with piggyBac, bottom) At 28 days post-injection, the livers were analyzed for GFP expression in the whole liver using a Biotek Cytation 3 (left panels). Fixed livers were sectioned and stained with an antibody that recognizes GFP to visualize expression at the cellular level (right panels).
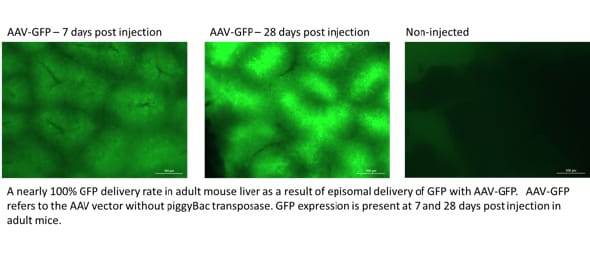
Figure 3: Livers analyzed for GFP expression in the whole liver
Applications And Technology Notes
Gene delivery can be classified into two approaches: viral and nonviral. Viral gene delivery systems consist of viruses that are modified to be replication-deficient, but which can deliver DNA for expression such as adenoviruses, retroviruses, and lentiviruses2. Non-viral approaches include nanoparticle, chemical and physical means of transfection. Many current gene therapy efforts have focused on the liver, due to advances in technology that allow for gene delivery at a high rate.
Viral approaches, including AVV (adeno-associated virus), are promising for therapeutic use in animal models and can effectively deliver transgenes to the liver in both mouse and rats. While AAV effectively delivers a transgene to the liver, the efficiency of integrating that transgene into the genome is very low, leading to loss of transgene expression over time. To improve efficiency, a combination approach has been developed using AAV and piggyBac transposon. The AAV is used to deliver the transgene as a transposon and also deliver the piggyBac transposase. piggyBac then uses a ‘cut and paste’ mechanism to stably integrate the transgene into the host genome. This approach has already been shown to be effective in delivering GFP into mouse hepatocytes at near 100% efficiency1.
This piggyBac-viral combination approach has also been demonstrated with adenovirus (Ad) and adeno-associated virus (AAV) as a potential therapeutic for cystic fibrosis through nasal delivery in mice. The piggyBac-viral system demonstrated efficient and persistent expression of the cystic fibrosis transmembrane conductance regulator (CFTR) gene following nasal delivery in mice and corrected chloride current in well-differentiated primary cultures of human airway epithelial cells derived from CF patients3. The in vivo delivery of viral genomes can create a model of viral replication in hosts such as mouse and rat which typically don’t support infection. For example, models of HBV and HCV have been developed using hydrodynamic injection in mice.
References
- Cunningham et al. (2015) Modeling Correction of Severe Urea Cycle Defects in the Growing Murine Liver Using a Hybrid Recombinant Adeno-Associated Virus/piggyBac Transposase Gene Delivery System. Hepatology, Vol. 62, No. 2.
- Zhang et al.(2012) In Vivo Gene Delivery by Nonviral Vectors: Overcoming Hurdles? 1. Molecular Therapy. 20 7, 1298–1304
- Cooney et al. (2015) Hybrid nonviral/viral vector systems for improved piggyBac DNA transposon in vivo delivery. Mol Ther. 2015 Apr;23(4):667-74
