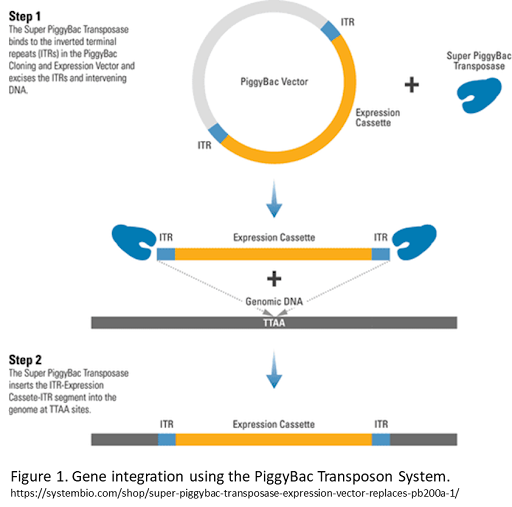
The piggyBac (PB) transposon system, originally identified in the Cabbage Looper moth, is a mobile genetic element that can be integrated into a host genome via the PB transposase “cut and paste” mechanism. To perform stable genomic integration, piggyBac transposon must be co-transfected with a piggyBac transposase protein or expression vector. The transposase then inserts the gene cargo and ITRs into the host genome (also known as transposition) (figure 1)1 at TTAA sites. The piggyBac transposon system offers numerous advantages over other gene delivery systems, and it is frequently used to generate genetically modified stable cell lines and transgenic animal models.
Hydrodynamic tail vein injection (HTVI) is an efficient strategy for in vivo gene delivery to mouse liver. HTVI avoids some drawbacks associated with other gene delivery techniques: adeno-associated viral vector can be immunogenic and targeted liposome delivery often fails to deliver adequate amounts of gene cargo.
The technique involves rapid injection (<5 seconds) of a large volume of aqueous solution (8-10% volume/body weight) containing nucleic acids. In early studies, gene delivery using HTVI often failed to achieve clinically significant protein expression levels. However, several groups have worked on optimizing the technique to increase protein expression in mice.
Physiologically, hydrodynamic tail vein injection achieves delivery of large volumes of solution by causing back flow from the hepatic vein into the liver. Additionally, the quick increase in blood volume also causes transient cardiac arrythmia, which resolves within minutes due to the rapid heart rate in mice. Accordingly, HTVI does not work as well in larger animals that have slower heart rates, including rats2. At the cellular level, the exact mechanism of gene cargo delivery is unknown. However, increased endocytosis has been described when cells encounter hypo-osmotic stress and increases in endocytic vesicles have been reported in hepatocytes following HTVI3.
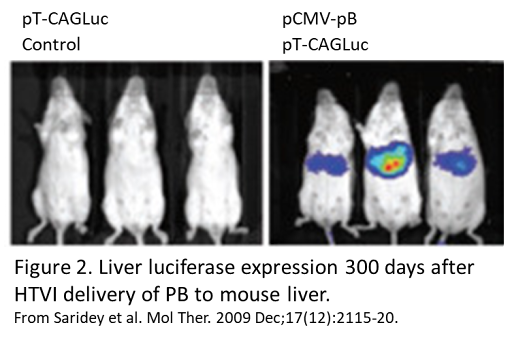
Several groups have used HTVI to deliver genes packaged in piggyBac to mouse liver. An early study reported robust luciferase expression for 6 months following HTVI delivery of piggyBac4. Another group, working with hemophilia A mice, reported that full length Factor VIII cDNA delivery resulted in stable expression of factor VIII protein for nearly 1 year, along with normalization of blood clotting5. Other studies have successfully shown luciferase expression for up to 300 days after injection (figure 2)6. This group also combined doxycycline-inducible gene expression with HTVI delivery of piggyBac and showed inducible gene expression for up to 120 days6.
Due to its ability to carry extremely large gene cargo (up to 200 kb pairs), the piggyBac platform is an extremely flexible technology capable of mediating long-term expression of simple to complicated gene editing systems. Paired with HTVI, it is an attractive option for manipulating gene expression in mouse liver.
References:
- Sato, M., Inada, E., Saitoh, I., Watanabe, S. & Nakamura, S. piggyBac-Based Non-Viral In Vivo Gene Delivery Useful for Production of Genetically Modified Animals and Organs. Pharmaceutics 12, doi:10.3390/pharmaceutics12030277 (2020).
- Sendra, L., Herrero, M. J. & Alino, S. F. Translational Advances of Hydrofection by Hydrodynamic Injection. Genes (Basel) 9, doi:10.3390/genes9030136 (2018).
- Crespo, A. et al. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther 12, 927-935, doi:10.1038/sj.gt.3302469 (2005).
- Doherty, J. E. et al. Hyperactive piggyBac gene transfer in human cells and in vivo. Hum Gene Ther 23, 311-320, doi:10.1089/hum.2011.138 (2012).
- Matsui, H. et al. Delivery of full-length factor VIII using a piggyBac transposon vector to correct a mouse model of hemophilia A. PLoS One 9, e104957, doi:10.1371/journal.pone.0104957 (2014).
- Saridey, S. K. et al. PiggyBac transposon-based inducible gene expression in vivo after somatic cell gene transfer. Mol Ther 17, 2115-2120, doi:10.1038/mt.2009.234 (2009).M
