Overview
Humanized liver rat and mice are created by ablation of native hepatocytes through either chemical or genetic alterations that cause hepatocyte damage. Human hepatocytes are transplanted into these animals, they repopulate the host liver replacing the endogenous liver with human cells. This type of humanization must be done in an immunodeficient or “SCID” animals so that the transplanted human cells are not rejected. The resulting humanized liver chimeric animal is an excellent model for studying in-vivo liver toxicity, drug transport, drug-drug interactions (DDI) and metabolism, as well as PK/PD (pharmacokinetics/ pharmacodynamics).
The evaluation of drug metabolism in fully mature human hepatocytes is important because the liver is the key organ for metabolism of xenobiotics and many hepatic enzymes are species specific. However, one of the major roadblocks for testing new drug candidates is the limited availability of human hepatocytes and the inability of stem cell-derived hepatocytes to fully mature in vitro. Liver humanized models enable discovery and toxicity testing on human hepatocytes in-vivo and provides a potentially unlimited source of fully differentiated mature human hepatocytes. Due to increasingly diverse chemical structures, it has become important to screen drug candidates for not only cytochrome P450 induced toxicity, but also other drug metabolism enzymes and drug-drug interactions (DDI). One recent study demonstrated, humanized liver mice could have predicted liver toxicity and prevented a failed clinical trial that led to the death of 5 patients by an investigational hepatitis B drug known as fialuridine (FIAU).
Traditional animal rodent models, such as Swiss -Webster mice and Sprague-Dawley rats, have sometimes failed to predict toxicity and pharmacokinetic/pharmacodynamic (PK/PD) behavior of xenobiotics in humans1. Conversely, hepatotoxicity in a rodent study does not always translate into similar toxicity in human subjects. Humanized liver rodent models are immunodeficient animals in which the native liver has been ablated and then engrafted with human hepatocytes, which proliferate to restore the functioning liver. These models offer an opportunity for advanced evaluation of new chemical entities for human specific hepatotoxicity. Humanized models are also useful for infectious disease studies such as hepatitis, which infect and replicate primarily in human hepatocytes but not in rodent2.
Humanized Liver Mouse Models
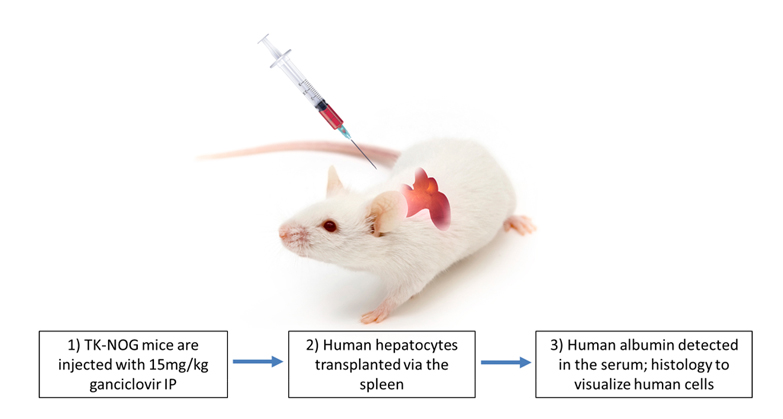
Hera has established humanized liver mice mouse models for metabolism and toxicology studies. Using the TK-NOG mouse background from Taconic liver ablation is induced by dosing the mice with ganciclovir. After ablation of murine hepatocytes, we transfer human hepatocytes to repopulate the liver and create the ‘humanized liver’ mouse model.
Creating Humanized Liver Mice
Confirming humanization by measurement of human albumin
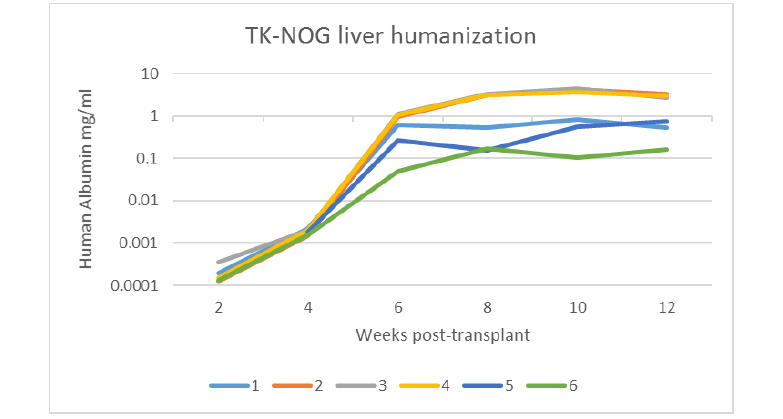
Human albumin is detected in the serum of TK-NOG liver-humanized mice. Blood was collected every 2 weeks starting at 2 weeks post-transplant of primary human hepatocytes in TK-NOG mice. Each line represents a single animal. 1-4, males. 5-6, females. Human albumin levels correlate with level of human chimerism (Hasegawa et al., 2011).
Detection of human hepatocytes within the chimeric TK-NOG liver.
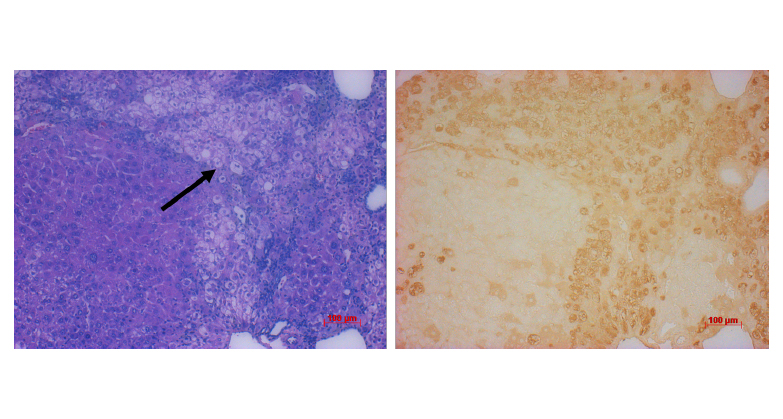
Left: H&E staining of liver sections confirms the presence of human cells, which are less eosinophilic than mouse hepatocytes and appear pale in comparison (arrow). Right: immunohistochemistry for human albumin (brown staining).
Working Toward a Humanized Liver Rat (HepatoRat)
Current research and development efforts focus on methods of liver ablation in the SRG rat to allow for high levels of human hepatocyte repopulation. Because the SRG rat demonstrates successful xenografts and allografts, we believe the HepatoRat will have several advantages over mice. These include more background toxicology data on the rat and ~10x more hepatocytes. Our genetically modified SRG rats is fully immunocompromised, similar to the NSG mouse. Rats are the preferred model in pre-clinical drug studies because their larger size facilitates procedures otherwise difficult in mice, including studies using instrumentation, blood-sampling, and surgeries. In rats, ten times the amount of tissue and serum can be collected per animal, enabling serial blood draws.
Contact us if you are interested in discussing our research and development progress or partnering opportunities.
Hera provides full service preclinical drug metabolism and toxicology CRO services to researchers in non-humanized rodent models as well. Below is an example study conducted by Hera.
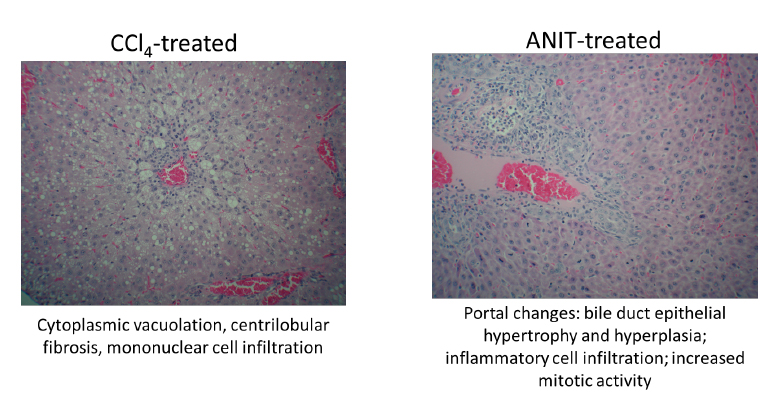
Example in vivo hepatotoxicity data (non-humanized rats): clinical pathology
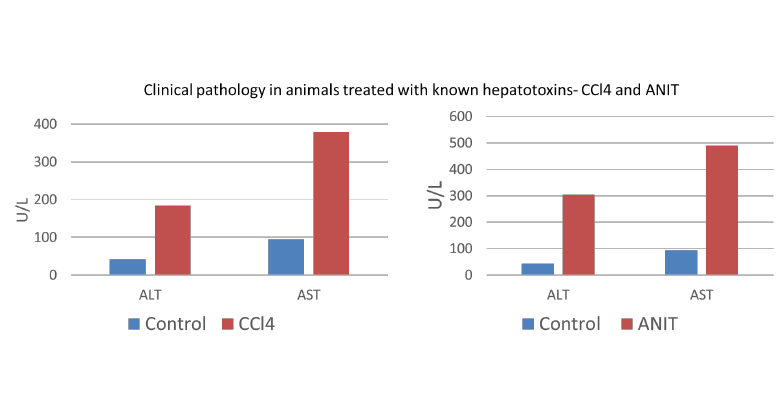
Alpha-naphthyl isothiocyanate/ANIT study (right): 75mg/kg dosed IP on days 0, 1, 2 (3 doses, analysis 48 hours post-dose). Blood collection via cardiac puncture for clinical pathology on day 3. Liver extracted, fixed, and processed for histopathology on day 3
CCl4 study (left): 5ml/kg dosed PO on days 0, 1 (2 doses, analysis 48 hours post-dose). Blood collection via cardiac puncture for clinical pathology on day 3. Liver extracted, fixed, and processed for histopathology on day 3
Example in vivo hepatotoxicity data (non-humanized rats): histopathology