Using Cas-CLOVER For Targeted Knock-Ins
Multiple mammalian cell expression systems are used to produce biotherapeutic proteins. However, development of recombinant mammalian cell lines is often delayed because of the unstable and variable transgene expression associated with random integration. To remedy this common gap in bioprocessing R & D, Hera offers an efficient strategy for site-specific integration of one or multiple copies of genes of interest (GOI).
Knock-In Strategy Using Cas-CLOVER
Our Cas-CLOVER system enables efficient insertion of a gene expression cassette at well-defined loci in mammalian cells, resulting in high yield homogenous protein production.
Knock-ins that are targeted to “safe harbor” genomic loci yield robust transgene expression without negatively affecting cellular characteristics. As such, the Hipp11 (H11) gene was targeted as it is well characterized as a “safe harbor” locus for gene knock-in within CHO-S cells. The Hipp11 (H11) locus, which is situated between the Drg1 and Eif4enif1 genes in multiple animal models, offers extensive potential for providing stable gene knock-in and high amounts of expression. [1, 2] The robustness of H11 has been demonstrated in mice, pigs, human embryonic stem (hES) cells, and induced pluripotent stem (iPS) cells. [1–3]
In collaboration with our partners at Demeetra, we established Cas-CLOVER targeted knock-in reagents and protocols at the Hipp11 safe harbor locus. Our proof-of-concept (POC) strategy outline is shown below and requires the knock-in of a not-so-small 4kb piece of DNA. (see figure below).
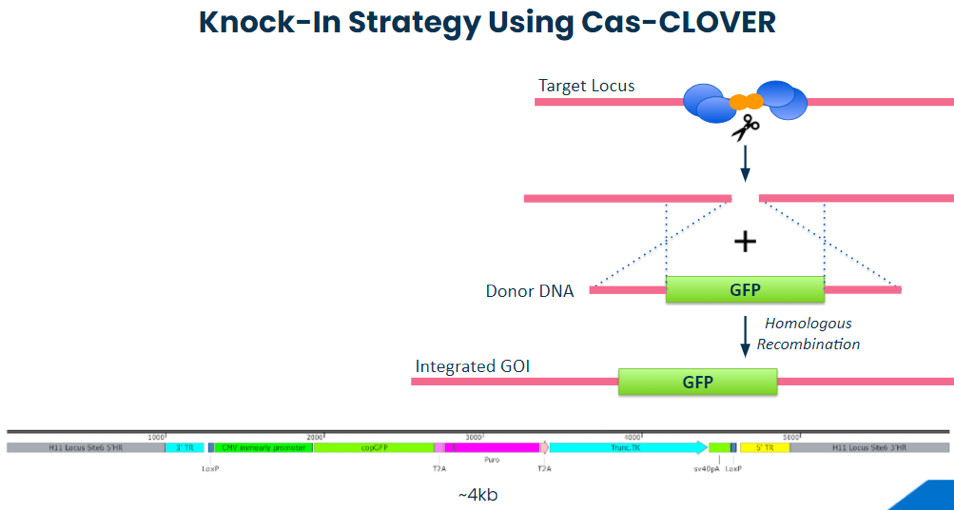
Diagram: knock-in strategy using Cas-CLOVER
The Cas-CLOVER system provides extremely efficient targeted knock-in (80-90%) in as little as two weeks, shown below. In comparison, random insertion yielded 0.68% in this study, and 1.32% GFP-positive cells in the CHO-S and HEK293T cells respectively.
Cas-CLOVER gRNA pairs at the GS locus (knockouts) and H11 safe-harbor site (knock ins) are completely validated, with targeted integration at the H11 safe harbor site confirmed by sequencing in multiple studies. In comparison, random insertion yielded 0.68% in this study, and 1.32% GFP-positive cells in the CHO-S and HEK293T cells respectively. The dimeric Cas-CLOVER is extremely accurate (i.e. 0% off-target mutagenesis) compared to up to 10% unwanted cutting with traditional CRISPR/Cas9.

Cas-CLOVER Bioprocessing Evaluation
Speak with Hera to learn more about acquiring rights to gene editing with Cas-CLOVER, for bioprocessing R & D, cell line engineering, reagent production, animal model creation, or human diagnostics.
Unlike CRISPR/Cas9, which has multiple licensing entanglements, Hera’s Cas-CLOVER can give you freedom to operate in a single license. We offer extremely competitive pricing for an initial evaluation period. Following successful evaluation, we offer highly affordable commercial licenses. Contact us at services@herabiolabs.com to learn more.
References
- Zhu F, Gamboa M, Farruggio AP, Hippenmeyer S, Tasic B, Schu ̈le B, et al. DICE, an efficient system for iterative genomic editing in human pluripotent stem cells. Nucleic Acids Res. 2014; 42.
- Simon H, Youn YH, Moon HM, Miyamichi K, Zong H, Wynshaw-Boris A, Luo L. Genetic Mosaic Dissection of Lis1 and Ndel1 in Neuronal Migration. Neuron. 2010; 68:694–709.
- Tasic B, Hippenmeyer S, Wang C, Gamboa M, Zong H, Chen-Tsai Y, et al. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc Natl Acad Sci. 2011; 108:7902–7. https://doi.org/10.1073/pnas. 1019507108 PMID: 21464299.
- Chi X, Zheng Q, Jiang R, Chen-Tsai RY, Kong L-J (2019) A system for site-specific integration of transgenes in mammalian cells. PLoS ONE 14(7): e0219842. https://doi.org/10.1371/journal.pone.0219842.
