The dissemination of tumor cells into systemic circulation is a critical area of cancer research, but it presents significant challenges. One obstacle is the lack of appropriate animal models that can support larger tumors and enable frequent, large volume blood collection. In this article, we explore the innovative research conducted by Ami Yamamoto and colleagues in Kevin Cheung’s lab at Fred Hutchinson Cancer Center.
Challenges with in vivo modeling of breast cancer dissemination
Aggressive cancers frequently exhibit necrotic tumor interiors, which is associated with poor clinical prognosis and the development of metastasis. [2-6] Despite this association, the precise mechanisms by which the necrotic core promotes metastasis are not fully understood. In this study, the research team employed SRG rats as a model organism to improve the detection of dissemination events and study molecular mechanisms that mediate the relationship between tumor necrosis and metastasis. [1]
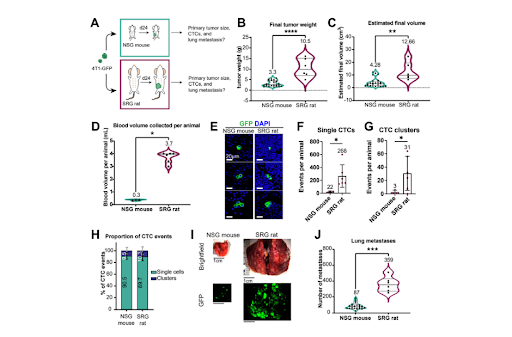
Supplemental Figure S1: Orthotopic transplantation into rats produce 3x larger tumors, 10x more CTCs, and 4x more lung metastases than into mice.
Breakdown of Figure S1:
- S1A) Experimental schema. 4T1 mouse mammary tumor cells labeled with membrane GFP (4T1-GFP) were orthotopically transplanted into a single #4 mammary fat pad of SRG rats (n=6) or NSG mice (n=24). Animals were sacrificed at 24 days post-transplantation.
- S1B-C) Final tumor weight and estimated final tumor volume per animal.
- S1D) Blood volume collected per animal.
- S1E) Representative micrographs of single CTCs and CTCs clusters from 4T1-GFP transplanted SRG rats. GFP denoted in green. DAPI in blue.
- S1F-G) Single CTC and CTC cluster abundance per animal. Individual blood samples from 8 mice were pooled into one tube for a total of n=3 samples. Events per individual animal are reported.
- S1H) Percentage of CTC events that were single CTCs or CTC clusters. Mean ± SD.
- S1I) Representative stereomicroscope images of lung metastases from NSG mice and SRG rats.
- S1J) The number of lung metastases determined by stereomicroscopy in transplanted SRG rats and NSG mice.
- Mean values shown on graphs. All P-values determined by Welch’s t-test.
Yamamoto et al. began by comparing tumor growth and metastasis in SRG rats versus NSG mice. They implanted the same volume of luciferase-tagged 4T1 mouse mammary carcinoma cells (4T1-Luc) into the mammary tissue of SRG rats or NSG mice. After 24 days of growth, as tumors approached the maximum allowable size for mice, tumors were removed and characterized. Rat tumor mass and volume was three-fold higher than mouse (Figure 1A-C). Additionally, the team was able to collect ten-fold more blood from rats versus mice, leading to a proportional ten-fold increase in the recovery of both single circulating tumor cells (CTCs) and CTC clusters (Figure 1D, F, & G). The ratio of CTC clusters to single CTCs did not differ between rats and mice, nor did their morphological appearance (Figure 1E & H). In keeping with higher numbers of CTCs, rats exhibited more lung metastases than mice, assessed using stereomicroscopy for GFP (Figure 1I & J). The authors concluded that, because the same number of 4T1 cells was transplanted in both SRG rats and NSG mice and because tumors were harvested at the same time, our direct comparison showed that the rat transplantation model increases the efficiency of detecting rare tumor dissemination events by 10-fold.
Having established the utility of the SRG rat, the authors proceeded to show that the development of necrosis is temporally related to dissemination of CTCs, in both rats and humans. Importantly, they report that dissemination occurs in the dilated blood vessels surrounding the necrotic tumor core, and that dissemination events are dependent on ANGPTL7 that is produced by tumor cells bordering the necrotic region. The authors suggest that ANGPTL7 could be a viable pharmacological target for the inhibition of metastatic events.
Benefits of the SRG rat model in CTC research
These findings make the SRG rat a promising model for studying CTC dissemination, and these results provide valuable insights into the molecular mechanisms underlying tumor dissemination and pave the way for the development of new therapies for breast cancer.
Figure 4: The SRG Rat lacks B, T, and NK cells.
Learn more about Hera’s SRG Rat Model
The SRG rat model has emerged as a promising tool for studying circulating tumor cells and metastasis, providing several advantages over traditional mouse models. Hera offers in vivo oncology studies utilizing the SRG rat in a wide variety of xenograft and PDX tumor models, allowing researchers to gain valuable insights into the molecular mechanisms underlying tumor dissemination.
Contact our team to learn more about our capabilities and expansive xenograft portfolio.
References:
- Yamamoto A, Huang Y, Krajina BA, et al. Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7. Proc Natl Acad Sci U S A. 2023;120(10):e2214888120. doi:10.1073/pnas.2214888120
- Fisher, E. R., Sass, R., & Fisher, B. (1984). Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer, 53(3 Suppl), 712–723. https://doi.org/10.1002/1097-0142(19840201)53:3+<712::aid-cncr2820531320>3.0.co;2-i
- Jimenez, R. E., Wallis, T., & Visscher, D. W. (2001). Centrally necrotizing carcinomas of the breast: a distinct histologic subtype with aggressive clinical behavior. The American journal of surgical pathology, 25(3), 331–337. https://doi.org/10.1097/00000478-200103000-00007
- Leek, R. D., Landers, R. J., Harris, A. L., & Lewis, C. E. (1999). Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. British journal of cancer, 79(5-6), 991–995. https://doi.org/10.1038/sj.bjc.6690158
- Bredholt, G., Mannelqvist, M., Stefansson, I. M., Birkeland, E., Bø, T. H., Øyan, A. M., Trovik, J., Kalland, K. H., Jonassen, I., Salvesen, H. B., Wik, E., & Akslen, L. A. (2015). Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget, 6(37), 39676–39691. https://doi.org/10.18632/oncotarget.5344
- Zhang, L., Zha, Z., Qu, W., Zhao, H., Yuan, J., Feng, Y., & Wu, B. (2018). Tumor necrosis as a prognostic variable for the clinical outcome in patients with renal cell carcinoma: a systematic review and meta-analysis. BMC cancer, 18(1), 870. https://doi.org/10.1186/s12885-018-4773-z
