Immunodeficient mice such as Nude, SCID and NSGTM have been instrumental for in vivo oncology. Due to the absence of immune response in these mice, tumor cells and tissue of human origin may be engrafted and tested for therapeutic efficacy. Mice were also used to spearhead the development of patient derived xenograft (PDX) models, which are generated by directly transplanting resected patient tumors. PDX models have demonstrated more appropriate tumor microenvironment and heterogeneity of tumor cells than cell line derived xenografts (CDX) and thus have been fundamental to research, drug development and precision medicine programs.1
However, due to relatively small size of tumors supported by mice, and differences in metabolism and physiology with larger research models such as rats, mouse models are not ideal for drug efficacy testing and downstream analysis2. The small size of tumors in mice limits the production of enough PDX material. Mouse PDX analysis and studies may often require 3-5 passages and 7-12 months or more3. The rat produces samples ten times larger than mice and is metabolically closer to humans making it the preferred model for pharmacokinetics (PK) and toxicology.
In addition, establishing appropriate xenograft models
in immunodeficient mouse strains faces two major limitations:
- Low take-rates: many xenografts show low, non- existent or inconsistent engraftment efficiency. Engraftment efficiencies in the 5-20% range are quite common .
- Inconsistent growth kinetics: There is a large variation in the growth rate of a xenograft in study animals, which makes it challenging to generate a cohort of animals with similar tumor sizes for an efficacy study.
In order to overcome the limitations of immunodeficient mouse models, Hera BioLabs developed the OncoRat SRG, a Sprague- Dawley rat with knockout mutations in the Rag2 and Il2rgamma genes resulting in depleted B-cells, T-cells, and NK-cells. Here we demonstrate OncoRat SRG tumor take rates and growth kinetics are superior to mouse models, thereby increasing the probability of study success rates and reducing animal usage numbers, ultimately making the OncoRat SRG a better choice for xenograft studies.
Improved Take Rate & Growth Kinetics
OncoRat SRG demonstrates high efficiency and desirable uniformity in a variety of tumor growth profiles. Many important human cancer cell lines, such as the VCaP prostate model and non-small cell lung cancer (NSCLC) PDX models have inadequate tumor growth in mouse models, hindering the ability to run efficacy studies.
VCaP was derived from a vertebral metastatic growth of a prostrate carcinoma. It is a desirable cell line for in vivo studies as it exhibits many of the characteristics of clinical prostate carcinoma. VCaP is a great model to study AR resistance as it expresses AR splice variants that have been shown to drive resistance to AR antagonists.4 Despite these advantages of the VCaP, due to its difficulty to grow in mice, the dominant in vivo model is the androgen responsive LNCaP, isolated from a human lymph node metastasis. VCaP cells exhibit very poor take rates (<20%) and high growth-rate variability in mice. The take rate would result in the need to inoculate many more animals than the study requires, but even if that approach were taken the growth kinetics are such that an efficacy study could not take place. OncoRat SRG demonstrates a 90%+ take rate and by 3 weeks post-inoculation, tumor growth permits efficacy studies (Figure 1). VCaP tumors surpass 13,0000 mm3 around 4-5 weeks post- inoculation (Figure 2).
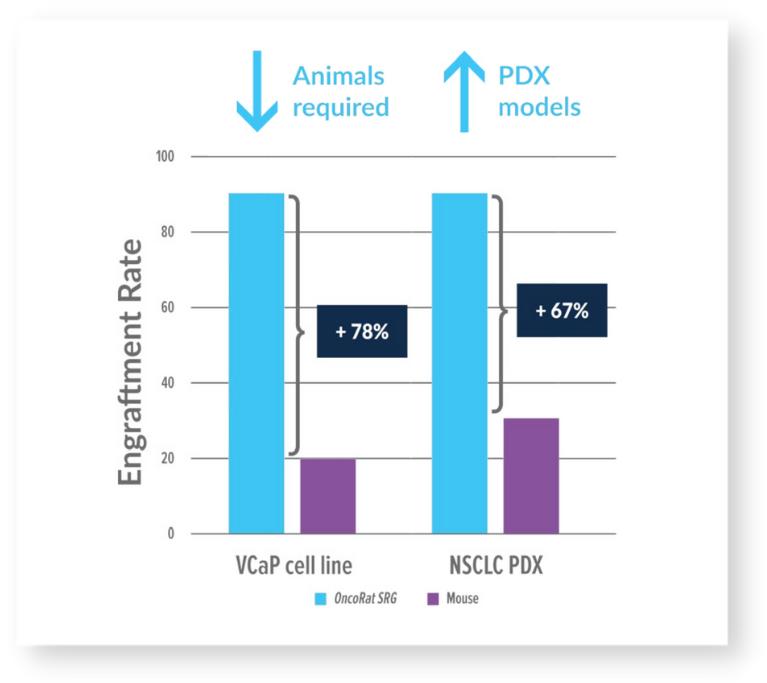
Figure 1: OncoRat SRG demonstrates increased tumor take rates
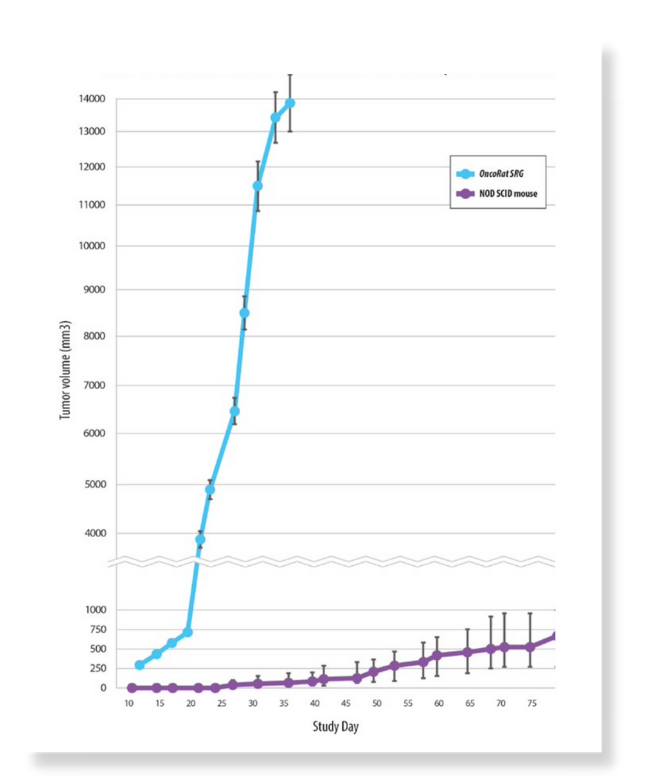
Figure 2: VCaP growth kinetics comparison OncoRat SRG and NOD SCID mouse
10x larger PDX tumors
Patient derived xenograft (PDX) studies initially focused on NSCLC indicate that the enhanced tumor growth seen for cancer cell lines in the OncoRat SRG translates into PDX models as well. Take rates are also improved meaning more PDX models can be created from tumor samples (Figure 1). Tumors grow 10x the size of mouse allowing for PDX studies including efficacy in a single passage (Figure 3), compared with 5 passages for NSCLC PDX in mice3. PDX growth kinetics are clearly enhanced in the OncoRat SRG as demonstrated in a passage 2 comparison with NSG mice (Figure 4).
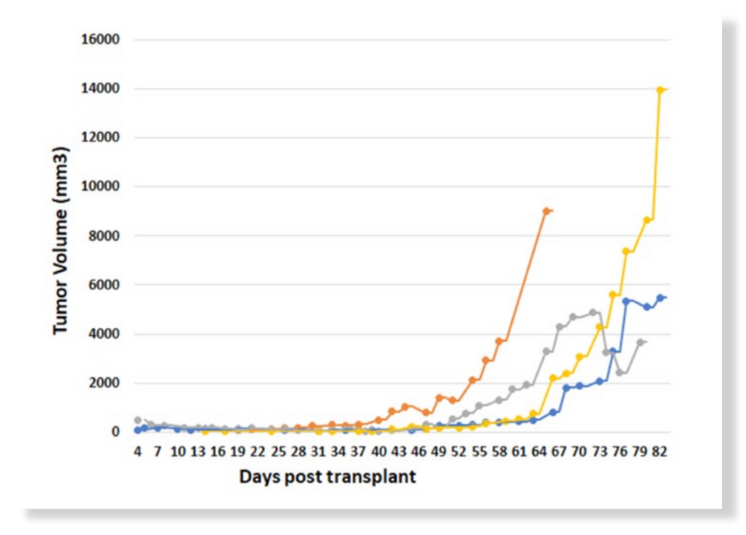
Figure 3: OncoRat SRG tumor kinetics for primary implants of NSCLC PDX
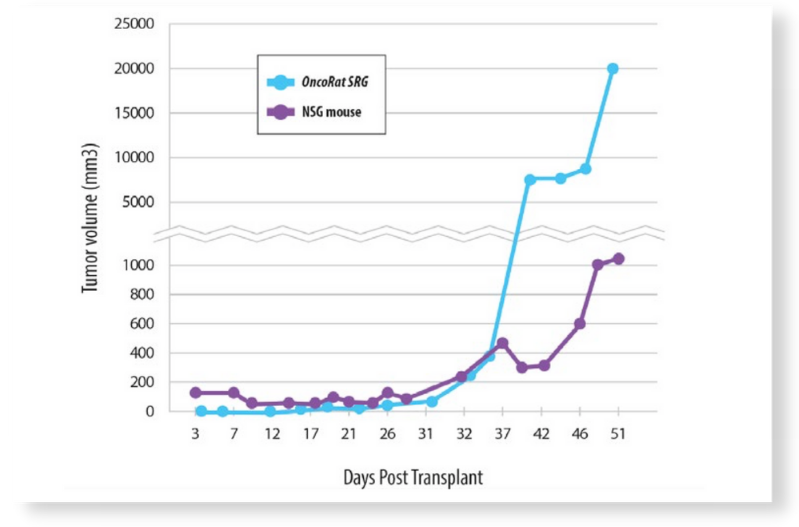
Figure 4: Growth curve comparison for passage 2 NSCLC PDX
Thus, OncoRat SRG improves several of the major pitfalls to PDX efficacy studies, the PDX establishment success rate and timeline from surgery to efficacy data.
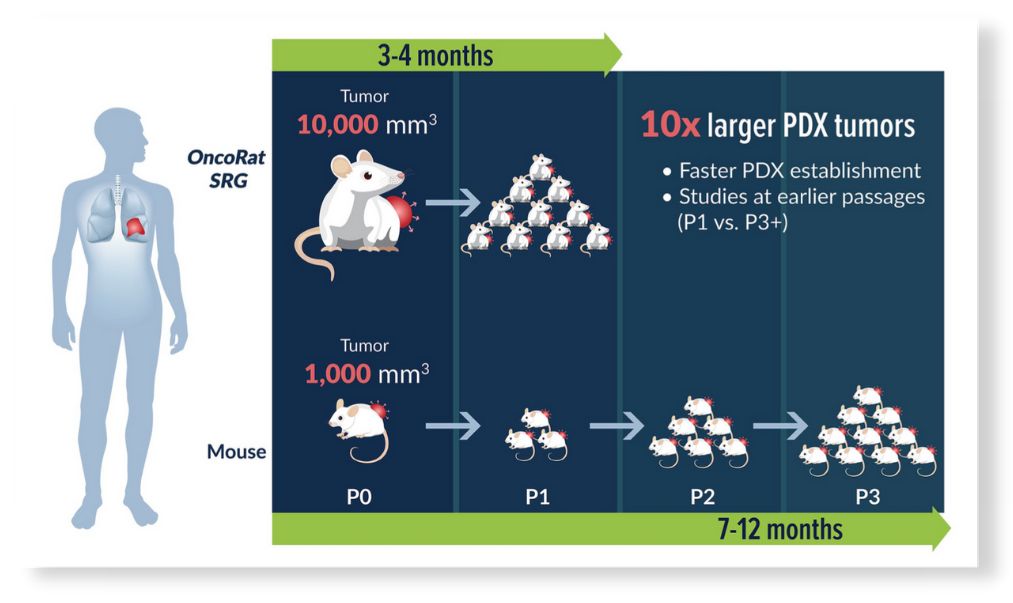
Figure 5: PDX study timeline & passage comparison
About Hera
Hera BioLabs’ foundational mission was to use cutting-edge genome editing technology to create new, precise, and more translation research models and provide high quality CRO services. Hera’s dimeric CRISPR system, Cas-CLOVERTM has little to no off-target mutations and can be combined with the piggyBacTM transposon to permit scarless gene editing. One of Hera’s first projects was to generate the OncoRat SRG. Our R&D pipeline includes humanizing the immune system and liver of the OncoRat SRG as well as other gene editing programs.
References
1. Oncotarget, 2015. 6(28):25619-30
2. Dis Model Mech. 2009 May-Jun; 2(5-6): 206–210
3. Frontiers in Oncology. 2017. v7. article 2.
4. European Urology. 2018 Apr;73(4):572-582.
NSG is a trademark of The Jackson Laboratory
