Engineered HepG2 Cells for Assessing CYP Mediated Metabolism
HepG2 is one of the most commonly used cell systems for in vitro screening, but HepG2 cells have low expression and activity of CYP enzymes which leads to missed or underestimated cytotoxicity of test compounds and/or metabolites
We have assembled a panel of HepG2 transgenic cell lines that each stably express one of the major human CYPs (originally published by Takeda Pharmaceutical Company Limited, Osaka, Japan) and utilized gene editing technology to create a CYP reductase (POR) knockout HepG2 cell line.
Used in appropriate assay panels, these cells can address a variety of questions pertaining to CYP mediated metabolism and drug/drug interaction
- CYP metabolism phenotyping can be accomplished directly with minimal or no use of inhibitors
- Direct or indirect determination of CYP inhibition
- CYP specific cytotoxicity
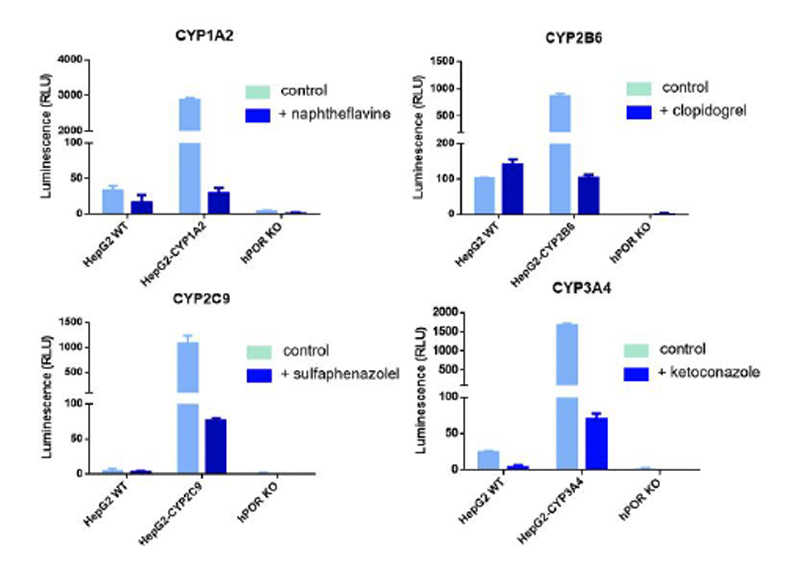
HepG2-CYP™ cells restore while reductase KO abolishes CYP activity
CYP activity in HepG2 WT, HepG2-CYP transgenic and HepG2-CYP reductase KO (POR) cells. CYP activity was measured with P450 –Glo™ assay kits. Specific CYP activity in transgenic cells was confirmed with CYP-selective competitive inhibitors, namely 5 µM α-naphthoflavone (CYP1A2), 1 µM clopidogrel (CYP2B6), 2 µM sulfaphenazole (CYP2C9), and 1 µM ketoconazole (CYP3A4).
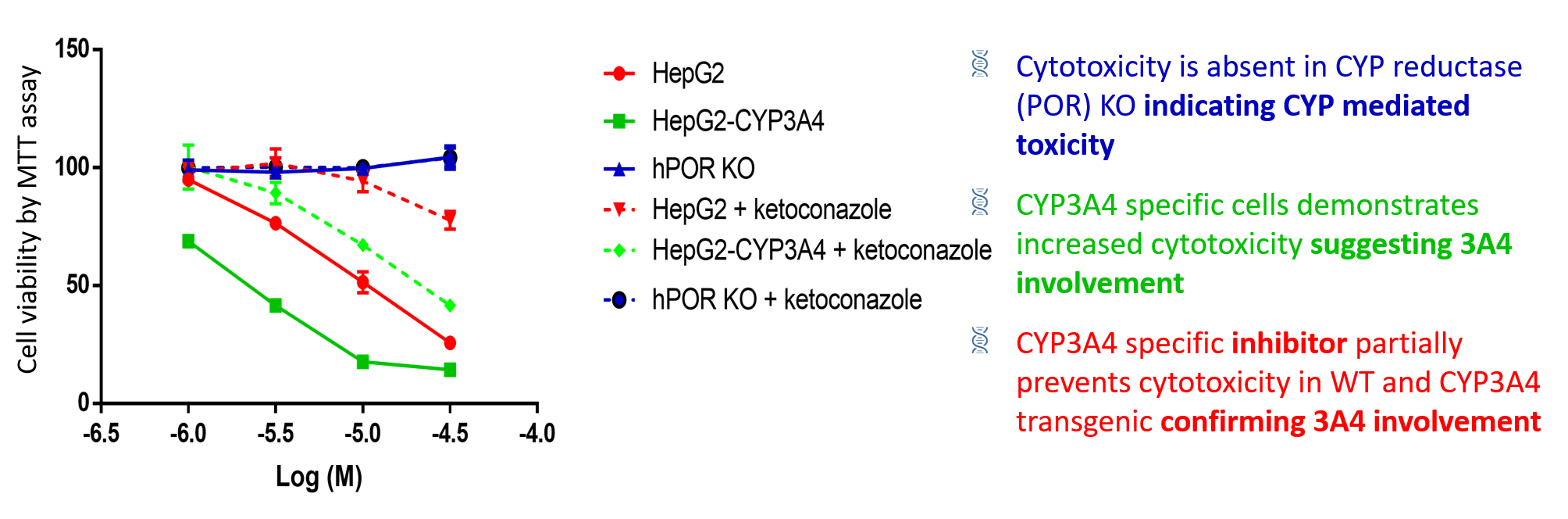
HepG2-CYP panel correctly identifies CYP3A4 role in Aflatoxin B1 cytotoxicity
Cells were treated with varied concentrations of aflatoxin B1 with or without 1 µM ketoconazole (CYP3A4 inhibitor). In cells treated with aflatoxin B1, concentration‐dependent cytotoxicity (based on loss of cell viability) was enhanced by the expression of CYP3A4 and suppressed by CYP reductase knockout. In HepG2 WT cells and HepG2-CYP3A4 cells, co- incubation with 1 µM ketoconazole partially protected against the toxic effect of aflatoxin B1.
HepG2-CYP™ Cell Panel
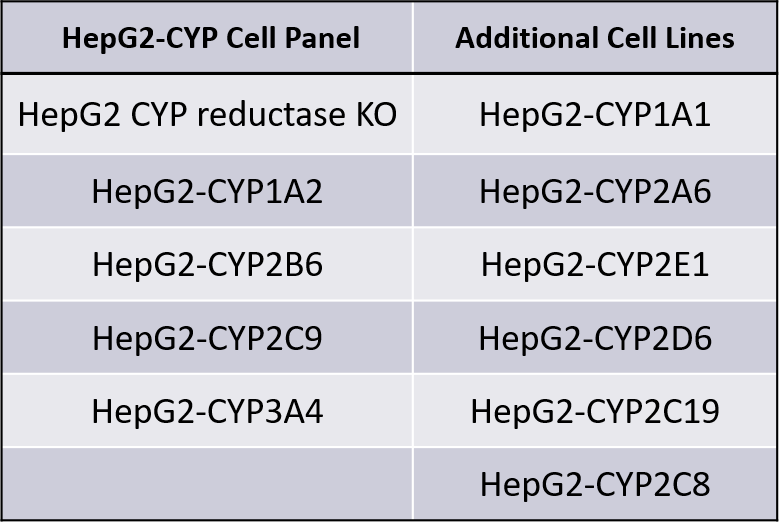
The HepG2-CYP cell lines are available as a panel or can be purchased individually
Hera provides in vitro screening services using the HepG2-CYP panel.
Services include:
- Cytotoxicity – cell viability (MTT & ATP)
- Metabolic stability & metabolite profiling
- CYP phenotyping and drug-drug interaction studies
Custom gene editing services (knockout, knock-in, and transgenic) to improve metabolic capability
of any cell line as well as further modifications to any of the cell lines from the HepG2-CYP panel.
Liver-like Functionality: HepG2 cells retain many of the functions of normal liver cells, including the production of several liver-specific proteins, like albumin and transferrin. With the added expression of the various cytochrome P450 (CYP) enzymes or the CYP null cell line, these cells are a useful tool for drug metabolism studies.
Ease of Culture: The HepG2 cells are relatively easy to culture in the lab and they grow well in standard culture media and under typical cell culture conditions.
Cancerous Origin: Since HepG2 cells were derived from a hepatocellular carcinoma, they are cancerous and can grow indefinitely in culture. They also exhibit some properties typical of cancer cells, such as resistance to some forms of cell death and changes in metabolism.
Genetic Stability: HepG2 cells are known to be genetically stable, which means their characteristics do not change significantly over time or across different laboratories. This is an important property for a cell line used in scientific research.
Applications in Research: HepG2 cells are widely used in various research fields, including toxicology, pharmacology, virology, cancer research, and more. For example, researchers often use these cells to study how drugs are metabolized in the liver, to test the toxicity of new drugs or chemicals, or to study the biology of liver cancer.
Please note that like all cell lines, HepG2 cells have limitations. While they have many liver-like properties, they do not perfectly represent a human liver. In some cases, primary human liver cells or more physiologically relevant systems (like liver organoids or tissue chips) might provide more accurate data.
