One of Hera’s key models, the SRG rat, has been consistently innovating and improving preclinical research since model generation. The SRG OncoRat®, developed using Hera Biolabs’ gene editing technology, is a SCID rat on the Sprague-Dawley background that harbors a double knockout for the Rag2 and Il2rg genes. Similar to NSG mice, the SRG demonstrates enhanced immunodeficiency, lacking B-cells, T-cells, and NK-cells. Cell lines that grow robustly in immunocompromised mice also grow robustly in the SRG rat, and cell lines that are challenging in mice, with variable take rates and inconsistent growth kinetics, grow consistently better in the SRG Rat.
Because of its larger size, the SRG rat also enables more frequent blood collection, larger tumor volumes, and, due to the Sprague-Dawley background, seamless transitions between toxicology, pharmacokinetics, and pharmacodynamics analyses. In fact, one distinct advantage to the SRG rat is that PK/PD studies can be done in the same animals as efficacy studies. This can help decrease the cost, timeframe, and number of animals used while increasing efficiency.
Other commercially available rat models, such as the Foxn Nude rat, are not as immunodeficient and therefore not as robust for tumor xenograft models. The nude rat models are athymic/T cell deficient, while our SRG rat is T, B, and NK cell deficient. With only T cell deficiency, spontaneous tumor regression can occur, which limits treatment time and contributes to variable tumor take rates and growth kinetics. l
Prostate Cancer Xenografts in Mice and Rats
In vivo tumor xenografts are a standard way to study tumor biology and test efficacy of pre-clinical therapeutics. Previous and ongoing studies have shown that human cell lines that grow robustly in mice also grow robustly in the SRG rat. In addition, cell lines that are challenging to grow in mouse models grow with improved tumor take rates and improved growth kinetics in the SRG. For example, VCaP and LNCaP prostate cancer cell lines can be very difficult to establish in immunocompromised mice, but they grow very well in the SRG (Figure 1).¹
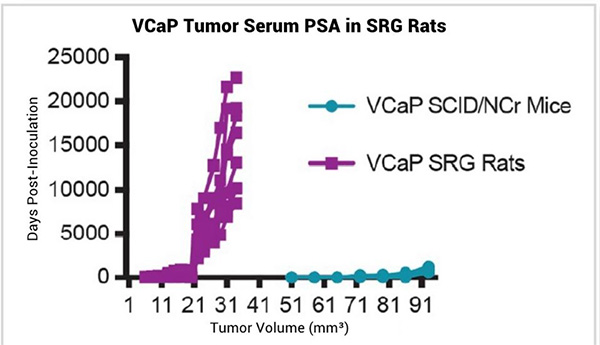
Figure 1
In addition to consistent VCaP tumor growth kinetics, serum prostate specific antigen (PSA) can be measured in the blood of VCaP tumor bearing SRG rats and levels directly correlate with tumor volume (Figure 2). PSA, the most widely used clinical marker of prostate cancer emergence and biochemical re-emergence, has value in pre-clinical research as well. Because of the larger blood volumes available, as well as the consistency between tumor volume and serum PSA level, the SRG rat is an ideal host for measuring PSA as a biomarker for VCaP growth.
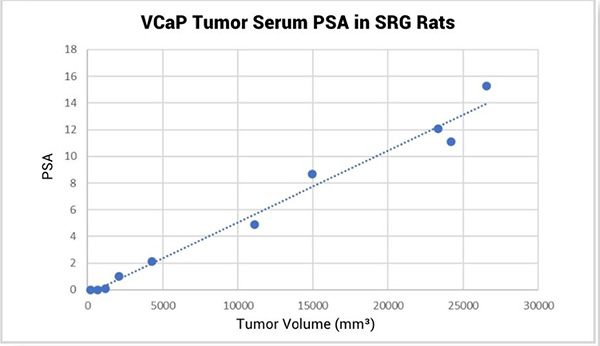
Figure 2
To further define the SRG rat, tumor histology was compared between VCaP tumors hosted in SRG rats or SCID/NCr mice (Figure 3). VCaP tumors were positive for androgen receptor (AR) and PSA staining, with expected cellular localization—nuclear with weak staining in cytosol for AR and extracellular staining for PSA. It is notable that VCaP tumors hosted by SRG rats have increased PSA production and stronger AR staining versus SCID/NCr mice.
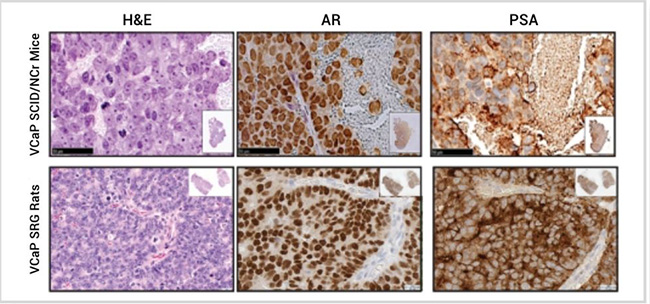
Figure 3
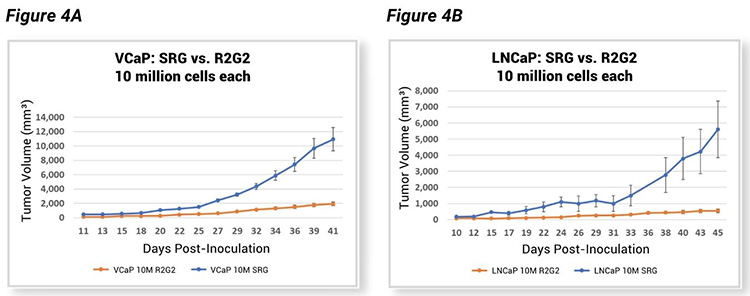
Hera compared tumor growth kinetics of the SRG rat and another immunocompromised model, the R2G2® mouse. Like the SRG rat, the R2G2 mouse is a Rag2 Il2rg knockout, devoid of B, T, and circulating NK cells. However, when testing VCaP and LNCaP cell lines in both models as subcutaneous tumor xenografts after inoculation with an equal number of cells into both species, tumors in SRG rats grew much faster (Figure 4, A-B). This data indicates there is a definite advantage to using the SRG as a host for tumor xenograft studies, considering the excellent tumor take rates and much larger tumor volumes.
In the OncoRat, Hera and collaborators have grown breast, colon, endometrial, glioblastoma, lymphoma, squamous cell carcinoma of the lung, non-small cell lung cancer, head and neck squamous cell carcinoma, ovarian, pancreatic, prostate, and renal cell carcinoma cell lines. Have a cell line that does not work in mice? Contact Hera today to start a pilot study for testing your cell line in SRG rats!
Testing Novel Prostate Cancer Therapeutic UT-34 in the OncoRat
Androgen receptor (AR) signaling is a key pathway in prostate cancer to support normal prostate function but also cancer growth. Targeting the AR signaling axis through AR antagonists has been a standard of care for prostate cancer treatment since the landmark discovery in the 1940s by Huggins and Hodges.² One staple AR targeting agent used in both research and in clinical practice is enzalutamide (MDV3100). Enzalutamide works through blocking androgen-AR binding, AR translocation to the nucleus, and AR binding to DNA with coactivators to alter transcription of downstream genes. However, most AR targeting therapeutics, including enzalutamide, require the AR ligand-binding domain (LBD), a protein domain that is commonly deleted in AR splice variants found in advanced prostate cancer.
Ramesh Narayanan led a University of Tennessee based team that tested a novel AR targeting agent, UT-34, that binds the AR activation function-1 (AF-1) domain.³ Independent of the AR-LBD, UT-34 has potential to work on a wide range of AR mutations, including splice variants such as AR-7 that have lost the LBD, and enzalutamide resistant cancers that have lost or mutated the LBD. UT-34 induces AR degradation via the ubiquitin proteasome pathway, a novel category of AR therapeutics that has great therapeutic potential. Narayanan et al. chose the SRG for their in vivo efficacy studies utilizing VCaP and enzalutamide resistant VCaP (VCaP-MVDR) tumor xenografts. Data shown (Figure 5, A–B) confirms tumor growth in SRG rats, and excellent therapeutic potential for UT-34 in both enzalutamide sensitive VCaP cells (Figure 5A) and enzalutamide resistant VCaP-MDVR cells (Figure 5B).
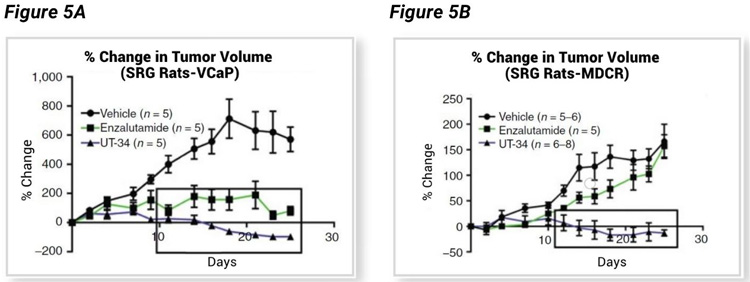
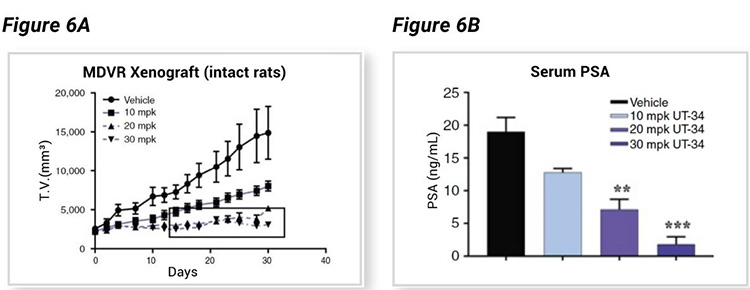
The xenograft tumor bearing SRG rats showed a dose-dependent decrease in tumor volume (Figure 6A) associated with a dose-dependent decrease in serum PSA level (Figure 6B), both enabled by consistent tumor growth rates and biomarker evaluation enabled by SRG rats and the therapeutic efficacy of UT-34.
An additional advantage utilized by Narayanan et al. is the predicted slower plasma clearance of UT-34 (less metabolism) in rats compared to mice. UT-34 is predicted to be rapidly metabolized in mice, which presented a challenge for in vivo efficacy studies due to the frequent dosing required to achieve target plasma levels. However, in rat as well as human liver microsomes, UT-34 is more metabolically stable than in mouse microsomes indicating the rat to be a better model for studying UT-34 versus mouse. In humans, UT-34 is expected to have similar, if not better pharmacokinetic properties than found in the rat. UT-34 is an incredibly exciting therapy, one whose pre-clinical efficacy is highlighted in the SRG rat.
Coming Soon at Hera: PDX and Standard of Care Options
There are limited patient derived xenograft (PDX) models available for prostate cancer research. This hinders research progress. Hera plans to commercialize a prostate cancer PDX bank to go alongside our non-small cell lung cancer (NSCLC) and ovarian cancer PDX establishment. These PDX tumors will be available to researchers to test their novel therapeutic in Hera’s SRG and immunocompromised mice.
It is a goal of Hera to expand our standard of care treatments available, so that individual researchers can easily and more readily compare their novel test article to established treatment regimes in the OncoRat. Having standard of care available will streamline the research needs of our clients, enabling Hera to increase research efficiency at a lower cost.
¹ Noto et al., 2020. The SRG rat, a Sprague-Dawley Rag2/Il2rg double-knockout validated for human tumor oncology studies. PLOS One. DOI.org/10.1371/journal/pone. 0240169.
² Huggins C, Hodges CV. 1941. Studies on prostatic cancer: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1:293-297.
³ Ponnusamy, S… Narayanan, R. 2019. Orally bioavailable androgen receptor degrader, potential next generation therapeutic for enzalutamide-resistant prostate cancer. Clin Cancer Res; 25(22).
